Research
Current research topics in the Rock Lab
Cells use an extensive toolkit of molecular motors to traffic material, to anchor internal components in fixed positions, and to control cell shape. Our work seeks to understand the molecular motors that drive biological movement and to use this knowledge to control cell motility.
We focus on myosin motors and the actin cytoskeleton as our model motility system. You have forty myosin genes in your genome that come from ten distinct classes, only a few of which are related to muscle. These unconventional, nonmuscle myosins are tuned to perform specific tasks within each and every cell. Since the emergence of the earliest eukaryotes, evolution has generated myosins that travel along distinct sets of tracks, step to a certain force with or without slipping, and travel in straight lines versus spiral paths around actin.
Many features of biological motility are still mysterious, but are functionally important. Some of the questions that we address include: How do motor proteins navigate the cytoskeleton, where an enormous number of tracks point in every direction? Can we uncover the cellular roadmap for motor traffic? Can we rewrite the cellular traffic rules?
Our current research topics include the following.
Mapping myosin traffic within the cell
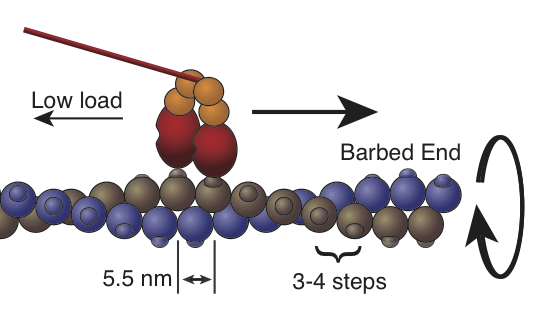
Our myosin-10 studies are an example of a “bottom-up” strategy for studying motor navigation, where we identify the individual players and examine them in isolated combinations. We have also developed “top-down” approaches, where we examine the traffic patterns of several types of myosins over a range of cellular states. We have developed what we call the “ex vivo motility assay” (Brawley PNAS 2009). Starting with cultured cells of nearly any variety, we extract the plasma membrane with a gentle detergent wash, exposing the actin cytoskeleton. We then apply fluorescently-tagged motor proteins (myosin-5, myosin-6, or myosin-10) and map their movements.
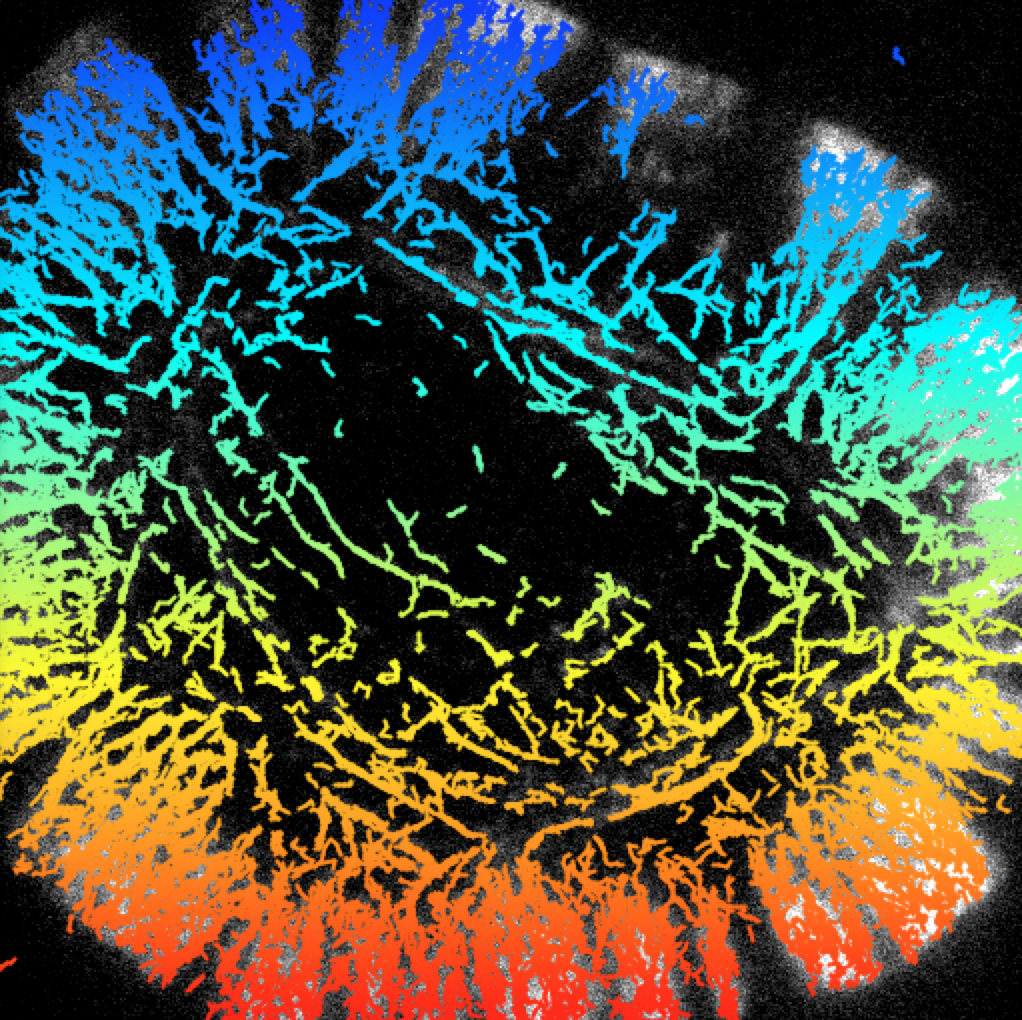
We find that the myosins segregate to different actin compartments depending on the myosin that is tested. Surprisingly, myosin-5 appears to be highly selective, moving along only small populations of actin and largely ignoring vast actin structures. In contrast, myosin-6 appears to walk along nearly all actin filaments. Thus, the actin cytoskeleton is not simply a uniform collection of tracks. Future work will focus on mapping cells with unusual actin structures.
Interplay between motors and filament tracks
Our mapping work suggests that myosins detect specific features of actin tracks. We use single molecule TIRF motility assays to discover these features. We teamed with the Kovar lab to investigate myosin motors in the context of dynamic actin filament polymerization, and found that different myosins can differentially detect actin filament age (Zimmermann Curr Biol. 2015). Future work will focus on the host of actin binding proteins that can modulate actin structure and provide additional myosin navigation cues.
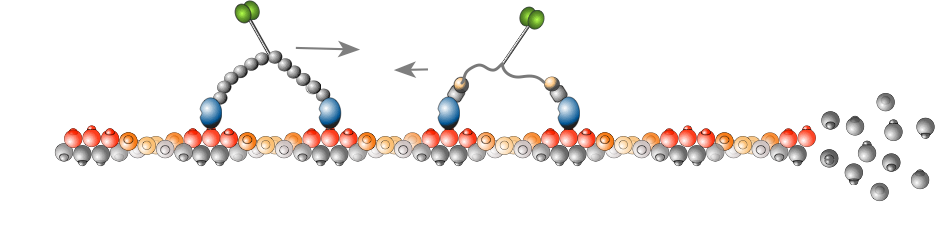
Optogenetic control of myosin motors
We have recently developed approaches to control the recruitment and activation of myosin-6 to specific sites within the cell (French PNAS 2017). Unlike many other approaches in this area, we focus our photoswitching efforts on the cargo adapters. This approach allows us to control unmodified, endogenous myosin-6, ensuring that force homeostasis is maintained both before and after we perturb the cell with light.
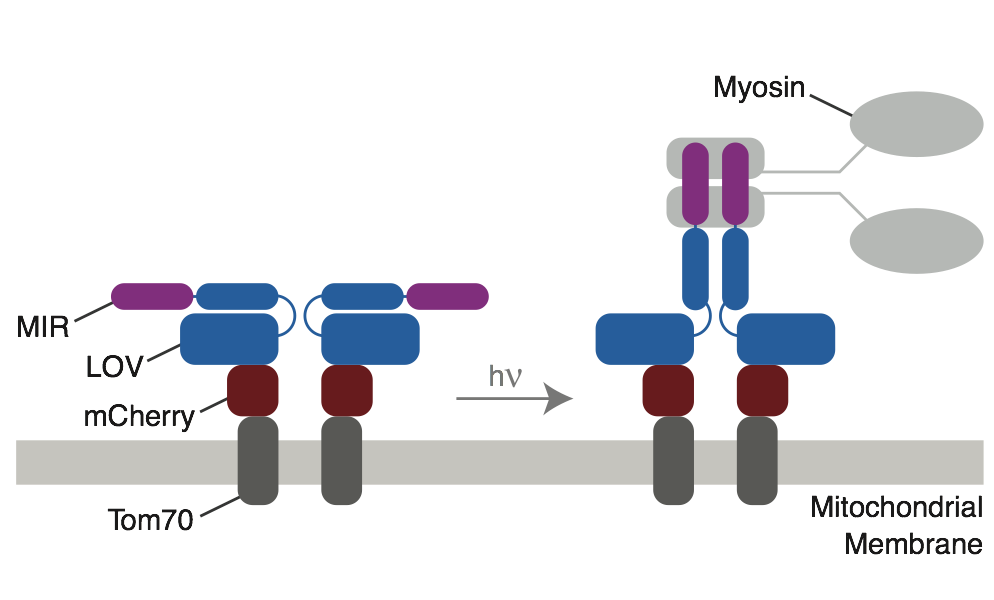
One of the powerful applications of this tool is to sequester myosin-6 at a distant site when it is required elsewhere. This sequestering is effectively a photoswitchable myosin-6 null (a photoswitchable Snell’s waltzer). This sequestering approach will allow us to dissect myosin-6’s role in numerous processes, including endocytosis, autophagy, Golgi morphology, and cell division.
The processive mechanics of nonmuscle myosin 2B
Nonmuscle myosin-2B (NM2B) is thought to set actin filament tension at the rear of migrating cells. To our great surprise, we discovered that NM2B walks processively along single actin filaments for several short steps (Norstrom, JBC 2010). This was the first time that processivity was observed in the class 2 myosins, which include the nonprocessive smooth, skeletal, and cardiac myosins found in muscle tissues. Indeed, nonprocessive motility is an essential feature of the muscle myosins, as it allows many motors to cooperate to drive high-speed motility. Therefore, NM2B demonstrates that the paradigm of muscle contractility does not even apply to every class 2 myosin, much less to the other “unconventional” myosins.